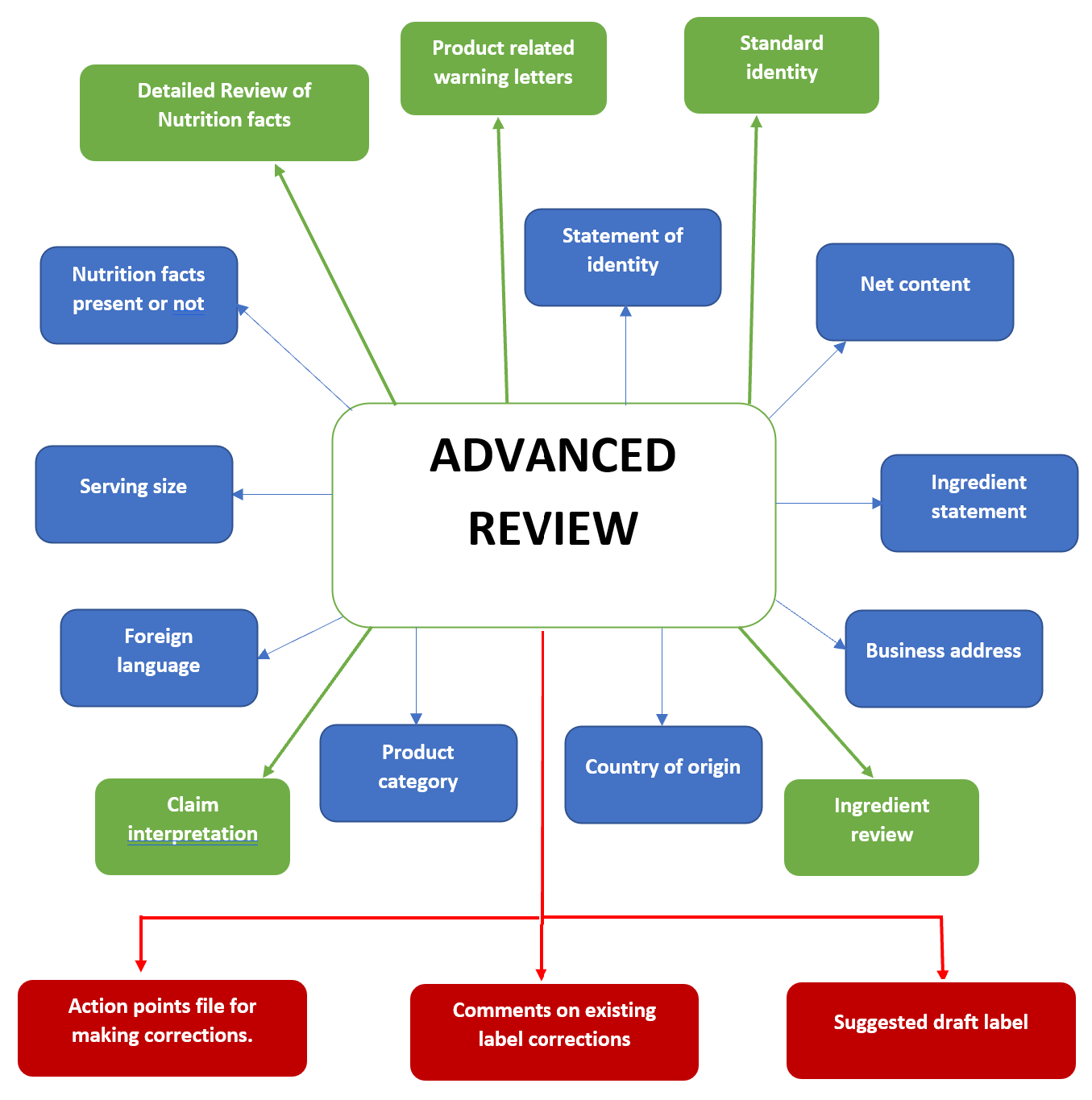
39 fda structured product labels
MTHSPL (FDA Structured Product Labels) - Statistics FDA Structured Product Label imprint attribute for shape text: 18077: BLA: Therapeutic Biologic Applications number for the MTHSPL drug: 15324: NDA: New Drug Application number for MTHSPL drug: 11751: DCSA: Controlled Substance Act designation code (e.g. 0,2,3n) 7193: MARKETING_EFFECTIVE_TIME_HIGH: Comirnaty Not Available - by Warner Mendenhall NDCs are listed per FDA Structured Product Label (SPL) document for the BLA licensed product. ... Pfizer has provided the following statement regarding the COMINARTY branded NDCs and labels: "Pfizer received FDA BLA license on 8/23/2021 for its COVID-19 vaccine for use in individuals 16 and older (COMIRNATY). At that time, the FDA published a ...
2010AB FDA Structured Product Labels Source Information FDA Structured Product Label UNII Code for Active Substance: 4093: COATING. FDA Structured Product Label imprint attribute for coating: 3034: SYMBOL. FDA Structured Product Label imprint attribute for symbol: 3032: DCSA. Controlled Substance Act designation code (e.g. 0,2,3n) 1975: NADA.

Fda structured product labels
Structured Product Labeling Resources | FDA The Structured Product Labeling (SPL) is a document markup standard approved by Health Level Seven (HL7) and adopted by FDA as a mechanism for exchanging product and facility information. FDALabel: Full-Text Search of Drug Product Labeling | FDA The source of FDALabel's data is the FDA's Structured Product Labeling (SPL) 1 archive 2,3, which stores labeling documents submitted by manufacturers. FDALabel is implemented as a secure three ... FDA Label Search The drug labels and other drug-specific information on this Web site represent the most recent drug listing information companies have submitted to the Food and Drug Administration (FDA). (See 21 CFR part 207.) ... Information about animal and human drug products can be found on these FDA Web pages: Animal and Veterinary Products, ...
Fda structured product labels. OnSIDES, side effects extracted from FDA Structured Product Labels OnSIDES, side effects extracted from FDA Structured Product Labels. May 17, 2022. May 17, 2022. OnSIDES (ON label SIDE effectS resource) is the newest member of the NSIDES family. The initial release (v01) of the OnSIDES database of adverse reactions and boxed warnings extracted from the FDA structured product labels. Outbreak Investigation of Salmonella: Peanut Butter (May 2022) | FDA The FDA, along with CDC and state and local partners, are investigating a multistate outbreak of Salmonella Senftenberg infections linked to certain Jif peanut butter products produced at the J.M ... IIS COVID-19 Vaccine Related Code | CDC The following vaccines and associated tradenames have been approved by the FDA under BLA License. They are listed separately because while they may represent the same formulations as the EUA authorized and labeled products listed above, the NDCs listed with the new BLA licensed tradenames in the FDA BLA approval or the FDA Structured Product Labels (SPL) are not currently being produced by the ... MTHSPL (FDA Structured Product Labeling) Source Information Health information suppliers can download drug labels in SPL format from DailyMed. Drug labeling on DailyMed is the most recent submitted to the FDA and currently in use. Sites Consulted. FDA Data Standards Council. Rockville (MD): Food and Drug Administration (US). Structured product labeling resources; [cited 2008 Oct 01].
SPL Xforms | FDA To register, please submit the following information via e-mail to spl@fda.hhs.gov: Attendee's first and last name. Name of your organization. E-mail address. Session name and date of training ... Structured Product Labeling (SPL) Terminology Files The NCIt-SPL terminology files provided here support the cooperative efforts of the Food and Drug Administration (FDA) and the National Cancer Institute's Thesaurus (NCIt) to develop terminology that facilitates the processing and review of SPL Terminology Files data. The efforts are described more fully on the Structured Products Labeling web ... Structured Product Labeling (SPL) | Data Conversion Laboratory Structured Product Labeling (SPL) is a standard used by the FDA community to facilitate the communication of drug labeling data reliability among various groups such as the FDA, hospitals, prescribing organizations, doctors, and the general public. SPL is an HL7 and ANSI approved standard. Since 2005, the FDA CDER division has provided guidance ... 2012AB FDA Structured Product Labels Source Information FDA Structured Product Label SET_ID code. 459423. NDC. National Drug Code corresponding to a clinical drug (e.g. 000023082503) 144687. DM_SPL_ID. DailyMed internal identifier for MTHSPL atom. 70092. LABELER.
Assessing the Impact of HL7/FDA Structured Product Label (SPL) Content ... The amount and quality of the SPL drug knowledge which has been released so far is assessed. All published labels were loaded into a relational database and classified to create vendor-independent descriptions. While SPL labels cover only 23% of RxNorm ... 2012AA FDA Structured Product Labels Source Information FDA Structured Product Label UNII Code for Active Substance: 9006: SHAPETEXT. FDA Structured Product Label imprint attribute for shape text: 8272: NDA. New Drug Application number for MTHSPL drug: 7563: OTC_MONOGRAPH_FINAL. FDA Structured Product Label OTC monograph status: 6843: OTC_MONOGRAPH_NOT_FINAL. Nsde | Fda Structured Product Labeling Resources ... Neither inclusion in the NSDE nor possession of an NDC number is a determination that a product is a drug as defined by the FD&C Act, nor does either ... Indexing Structured Product Labeling | FDA Indexing Structured Product Labeling. This guidance explains that FDA's Center for Drug Evaluation and Research (CDER) and Center for Biologics Evaluation and Research (CBER) will index the ...
Structured Product Labeling - Food and Drug Administration Structured Product Labeling (SPL) is a document markup standard approved by Health Level Seven (HL7) and adopted by FDA as a mechanism for exchanging product and facility information. The openFDA drug product labeling API returns data from these submissions for both prescription and over-the-counter (OTC) drugs. The labeling is broken into ...
Package Type | FDA In this section: Structured Product Labeling Resources ... Electronic Animal Drug Product Listing Directory; Equivalence Codes; Flavor; Geopolitical Entities, Names, and Codes (GENC) ...
PDF Structured Product Labeling Implementation Guide for FDA Drug ... SPL Implementation Guide for FDA Drug Establishment Registration and Drug Listing v2.0 3 Terminology: None . SPL location: This information is in the beginning of the SPL file.. XML details: The instructions at the start of SPL are the same for every SPL document (the encoding set is dependent on the character encoding used in the SPL) and are in the following form:
2011AA FDA Structured Product Labels Source Information IMPRINT_CODE. FDA Structured Product Label imprint attribute for code. 14314. ANDA. Abbreviated New (Generic) Drug application number for the MTHSPL drug. 11049. BLA. Therapeutic Biologic Applications number for the MTHSPL drug.
FDA warns consumers to not eat certain Jif peanut butter because of ... Recalled products include the products below with lot codes 1274425 - 2140425. Lot codes are included alongside best-if-used-by date," according to the company's recall notice posted by the FDA.
Drug Labeling Overview - Food and Drug Administration Drug manufacturers and distributors submit documentation about their products to FDA in the Structured Product Labeling (SPL) format. The openFDA drug product labeling API returns data from this dataset. The labeling is a 'living document' that changes over time to reflect increased knowledge about the safety and effectiveness of the drug.
Structured Product Labeling Validation Rules Guidance for Industry - Indexing Structured Product Labeling (Final) Guidance for Industry: Self-Identification of Generic Drug Facilities, Sites, and Organizations ... 4 Drug Labeling, Listing ...
Structured Product Labeling Improves Detection of Drug-Intolerance Issues Introduction and Objective. The HL7 Structured Product Labeling (SPL) standard 1 implemented by the FDA uses the HL7 Reference Information Model (RIM) 2 to represent the chemical and physical nature of medical products and their safe and effective use. While not all of this content is available today, we enrich the 3704 available SPLs with knowledge from the SPL terminology sources, including ...
USFDA structured product labeling update - June 2020 July 12, 2020 admin 0. In month of June 2020, USFDA updated header of Structured Product Labeling (SPL) XML: Updated xml-stylesheet reference. Updated the schemaLocation of the urn:hl7-org:v3 namespace.
Structured Product Labeling - Wikipedia Structured Product Labeling (SPL) is a Health Level Seven International (HL7) standard which defines the content of human prescription drug labeling in an XML format. The "drug labeling" includes all published material accompanying a drug, such as the Prescribing Information which contains a great deal of detailed information about the drug.
DailyMed - FDA Resources: SPL, Other Prescription Drug Labeling ... FDA's Structured Product Labeling Resources. Structured Product Labeling (SPL) is the standard format for electronic submission of the content of labeling. For SPL resources (including industry data standards for SPL), see FDA's SPL Resources page and the "Structured Product Labeling Resources" heading on FDA's Prescription Drug Labeling ...
UMLS Metathesaurus - MTHSPL (FDA Structured Product Labels) - Synopsis SPL is a document markup standard approved by Health Level Seven (HL7) and adopted by the FDA as a mechanism for exchanging medication information. Metathesaurus Scope. MTHSPL includes drug product and active substance terminology used in Structured Product Labels. MTHSPL contains approximately 158,821 drug products and 21,070 substances.
FDA Label Search The drug labels and other drug-specific information on this Web site represent the most recent drug listing information companies have submitted to the Food and Drug Administration (FDA). (See 21 CFR part 207.) ... Information about animal and human drug products can be found on these FDA Web pages: Animal and Veterinary Products, ...
FDALabel: Full-Text Search of Drug Product Labeling | FDA The source of FDALabel's data is the FDA's Structured Product Labeling (SPL) 1 archive 2,3, which stores labeling documents submitted by manufacturers. FDALabel is implemented as a secure three ...
Structured Product Labeling Resources | FDA The Structured Product Labeling (SPL) is a document markup standard approved by Health Level Seven (HL7) and adopted by FDA as a mechanism for exchanging product and facility information.



_(7396780726).jpg/510px-Looking_at_Food_Labels_(098)_(7396780726).jpg)











Post a Comment for "39 fda structured product labels"